-
2025-08-25
Hasten Announces Topline Results of HST101 (Lerodalcibep) Phase 3 Clinical Trial in Chinese Patients with Hypercholesterolemia
-
2025-02-13
LIB Therapeutics Announces FDA Acceptance of Biologics License Application for Lerodalcibep to Lower LDL-Cholesterol Across Broad Patient Population
-
2024-12-19
LIB Therapeutics Submits a Biologics License Application to FDA for Lerodalcibep for the Treatment of Adults with Elevated LDL-Cholesterol
-
2024-11-18
Hasten Announced the First Subject was Enrolled and Dosed in the Phase III Clinical Trial of Lerodalcibep
Hasten Biopharma Announced that the Clinical Trial Application for Lerodalcibep Has Been Accepted by the National Medical Products Administration's CDE
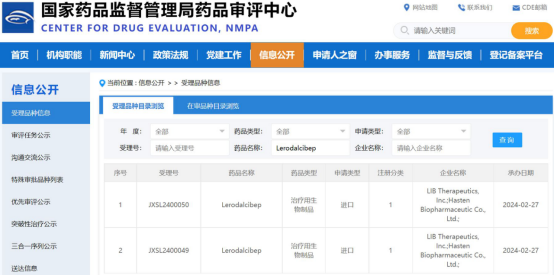
On Feb. 27th, 2024, Hasten Biopharmaceutical Limited Co., Ltd. (hereinafter as Hasten Biopharma) announced that the clinical trial application (IND) for Lerodalcibep, a novel third-generation PCSK9 inhibitor, has been accepted by the Center for Drug Evaluation(CDE) of the National Medical Products Administration (NMPA) for the treatment of patients with hypercholesterolemia and further reduction their low-density lipoprotein cholesterol (LDL-C) levels.
Summer XIA, CEO of Hasten Biopharma, said:“after obtaining the exclusive rights for the development and commercialization of Lerodalcibep in Greater China, we immediately started the clinical trial application in China, submitted materials promptly and obtained acceptance within 6 months. This surely manifests the spirit of 'Hasten speed' and the company's commitment to promote the clinical research process of innovative drugs in China and further solidifies the strategic path of Hasten Biopharma's whole industry chain development and multi-pipeline progress, which will better assist the realization of the strategic goal of 'Healthy China 2030' and the National Health Plan for 'the 14th Five-Year Plan'.”
Dr. Lisa LIU, Vice President and Head of Medical Department of Hasten Biopharma, said, "Data from Phase III clinical trials abroad have demonstrated that Lerodalcibep, a novel third-generation PCSK9 inhibitor, can sustainably reduce LDL-C when administered as a monthly subcutaneous one-shot dose. Meanwhile, the results of existing stability experiments show that it possesses superior stability at room temperature. We will actively cooperate with the regulatory authorities in the review of Lerodalcibep to advance the clinical trial process, so that it can provide more treatment options for patients with atherosclerotic cardiovascular disease (ASCVD) and/or those at very high or high risk of ASCVD as soon as possible."
The acceptance of the Lerodalcibep clinical trial application (IND) by the NMPA was based on the integrated data dossier provided by LIB Therapeutics,Inc., including CMC, non-clinical and clinical studies completed in the U.S. and Europe , as well as the clinical development plan in China proposed by Hasten Biopharma.
Lerodalcibep, a novel third-generation PCSK9 inhibitor, is the first in-licensing innovative medicine in development of Hasten Biopharma, which synergies with the company’s seven existing original medicines to strengthen its portfolio in the chronic and acute critical diseases. In the future, Hasten Biopharma will work closely with more partners to meet the growing unmet needs from Chinese patients and provide high-quality healthcare solutions persistently.
Notification: The drug under development related to this article has not yet been approved in China. Hasten Biopharma does not recommend the use of any unapproved drugs.
Reference:
- Laufs U, Blüher M, Isermann B. Third generation PCSK9-Inhibitors. Eur Heart J. 2023 Aug 28:ehad566. doi: 10.1093/eurheartj/ehad566. Epub ahead of print.
- Raal F, Fourie N, Scott R, Blom D, De Vries Basson M, Kayikcioglu M, Caldwell K, Kallend D, Stein E; LIBerate-HeFH Investigators. Long-term efficacy and safety of lerodalcibep in heterozygous familial hypercholesterolaemia: the LIBerate-HeFH trial. Eur Heart J. 2023 Aug 28:ehad596. doi: 10.1093/eurheartj/ehad596. Epub ahead of print.
公司地址:这里是公司地址后期需要替换设计占位后台可替换
企业邮箱:infu@singlera.com.cn
联系电话:+86-8888-8888
商务联系:BD@singlera.com.cn
人力资源:HR@singlera.com.cn








